Abstract
Background Tyrosine kinase inhibitors (TKIs) have significantly improved clinical outcomes of patients (pts) with Ph/BCR-ABL1-positive (Ph/BCR-ABL1+) acute lymphoblastic leukemia (ALL). The second-generation TKIs result in a better response rate and survival, compared to the first-generation. Flumatinib is a novel second-generation TKI and has been approved in 2019 in China. It has been demonstrated better efficacy compared to imatinib in clinical trials of CML, but few reports are available in ALL. Herein, RJ-ALL2020.2A trial is the first registered clinical trial of flumatinib in Ph/BCR-ABL1+ ALL to evaluate its efficacy and safety in adult pts.
Methods In this prospective phase II study, pt eligibility mainly includes: 18 ≤ age < 65 years; newly diagnosed Ph/BCR-ABL+ ALL; and adequate organ function. Once the diagnosis is confirmed, combination of flumatinib (600mg/day) and VIP-based chemotherapy regimen (Vincristine/Idarubicin/Prednisone) is administered promptly. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is recommended to all eligible pts. Central nervous system (CNS) prophylaxis is regularly performed by intrathecal injection of methotrexate, cytarabine, and dexamethasone after remission induction course. Multiparameter flow cytometry (MFC) and real-time quantitative polymerase chain reaction (RT-qPCR) are both used to monitor minimal residual disease (MRD). The primary endpoints are MRD clearance, progression-free survival (PFS) and overall survival (OS). (Clinical Trial Registration Number: ChiCTR2100042248)
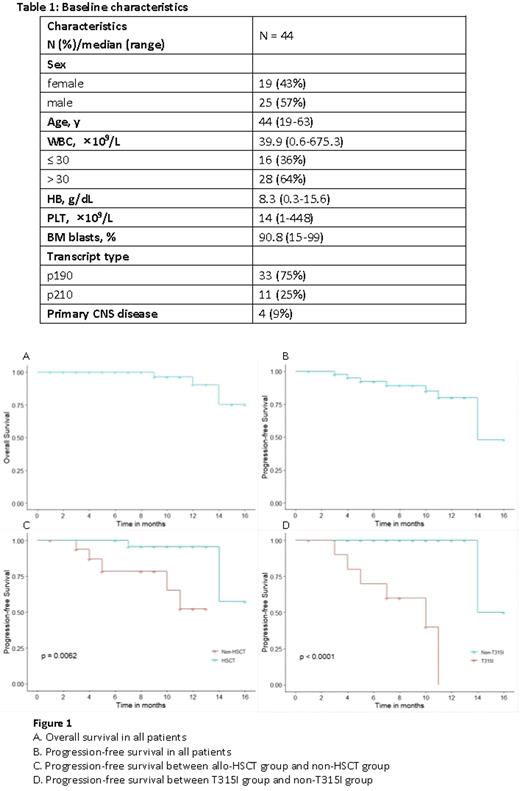
Results From August 2020 to June 2022, 44 adult pts with de novo Ph/BCR-ABL1+ ALL were enrolled. Median age was 44 years (range, 19-63). Median WBC count was 39.9×109/L (0.6-675.3), and 4 pts had primary CNS leukemia (Table 1).
There was no early death during the induction course. 41/44 (93.2%) pts achieved CR at the end of induction. MRDneg (<0.01%) rates by MFC were 65.9% (27/41) and 76.9% (30/39), while the CMR rates were 35% (14/40) and 50% (19/38) at the end of induction and 3 months, respectively. With a median follow-up of 10 months (range, 1-16), the estimated 1-year PFS and OS rates were 79.9% and 90.3%, respectively (Figure 1A, B). Among the 41 survived pts, 34 pts were in continuous remission, 2 were refractory and 5 were relapsed. Among 3 deaths, 2 died of disease progression, and 1 of pulmonary infection post-HSCT. 24 pts received allo-HSCT with a median time of 6 months (range, 3-10) from diagnosis (12 with haploidentical donors and 12 matched donors), the median age was 38 years (range, 20-60). Before HSCT, MRDneg rate was 75% (18/24), and CMR rate was 66.7% (16/24). Pts who underwent HSCT had a relatively better outcome, with 1-year PFS rate significantly higher than non-HSCT group (95.7% vs. 52.2%, p=0.006) (Figure 1C), and 1-year OS rate tending to be higher (100% vs. 74.1%, p=0.145). Among 40 pts evaluable for ABL1 mutations, 14 pts were mutated. T315I was most frequently identified, accounting for 71%, followed by Y253H (29%), E255V (14%), E255K (7%), T267A (7%). Pts with T315I mutation showed a poorer outcome than non-T315I mutation (no mutation or other mutations), with 1-year PFS rates 0% vs. 100% (p<0.001) (Figure 1D), 1-year OS rates 55.6% vs. 100% (p=0.06), respectively.
Meanwhile, the combination of flumatinib and chemotherapy was well-tolerated. The adverse events of grade 3-4 were mostly hematologic, which may be related to induction chemotherapy. Other side effects such as gastrointestinal symptoms and hepatic toxicity were grade 1-2, which could be recovered soon after symptomatic management. No pt discontinued flumatinib due to toxicity.
Conclusion The combination of the second-generation TKI flumatinib and chemotherapy is quite effective and safe in Chinese adult pts with newly diagnosed Ph/BCR-ABL1+ ALL. This clinical trial is still ongoing, and the long-term follow-up data will be further investigated.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Flumatinib is used for adult patients with Philadelphia chromosome/BCR-ABL1-positive acute lymphoblastic leukemia
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal